Discussions about LEDs’ pros and cons in the sign industry have perpetuated the issue of light-generation efficiency. Worldwide, fluorescent-lamp manufacturers are working on even more efficient lamps and materials.
The neon industry hasn’t ignored this demand, contrary to some claims made by the "silicon Mafia." Many new, more efficient (brighter at the same current) colors have been introduced to the sign industry in the last two years. This month, I’ll shed some light on how fluorescent powders work and what new materials may offer.
Simply put, neon-sign tubing can be divided into coated, uncoated and colored glass. Coating differences permit the output of multiple colors with the same type of gas fill — usually argon with a tiny mercury droplet. But how does the coating, a very fine layer of (usually) white powder, generate all the available colors?
These powdery substances transform invisible UV light into visible light of a particular color through a process called fluorescence. An electric-gas discharge of mercury vapor provides the light.
Seeing the light
To understand fluorescence, we must briefly examine quantum and solid-state physics. Light comprises electromagnetic waves in a certain wavelength; each pure color of the rainbow represents a distinct wavelength. Red light has a longer wavelength than blue light; the shorter the wavelength, the higher the wave’s energy.
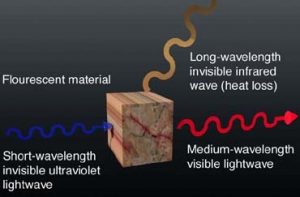
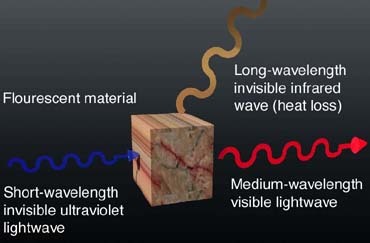
Fig. 1 symbolizes the principle of fluorescence: UV lightwaves possess very high energy (and, therefore, short wavelengths), which, when absorbed by fluorescent material, charges the crystal with energy. Instantly (in roughly 0.000000000001 seconds), a visible lightwave with lower energy (with a longer wavelength) radiates from the crystal. Shortly thereafter, the remaining energy charge emits as an invisible heat wave (with a very long wavelength), which leaves the crystal in its original state, unchanged.
Atomic electrons inside the solid-state fluorescent crystal carry out the fluorescence process. In an atom, electrons take different orbits around the nucleus, and each orbit represents a specific energy level carried by the electron (Fig. 2a). The larger the orbit, the higher the electron’s energy level.
If a UV lightwave hits a specific electron, its energy lifts the electron into a higher orbit, and consumes and absorbs the light. This leaves the former position void (Fig. 2b). The whole atom is now "excited" — it carries higher energy than in its normal state. If an electron on a higher orbit falls back into a void, a lightwave, which bears the same wavelength and color as the energy difference, is emitted to complete the exchange of light and energy (Fig. 2c).
When the energy difference during absorption and emission is the same, the emitted light has the same wavelength as the absorbed light. In the case of UV light, the result would be invisible. Therefore, the flourescent material must complete another process.
Filling the void
Visible light is generated from UV lightwaves when the emitted energy is lower than the absorbed energy. Atoms inside any solid-state body, such as the fluorescent powder, aren’t free, but are always close to their neighbors. Thus, the energy from an excited atom transfers to its surrounding environs. A traveling electron will fill a void near the excited atom.
If the nearby void’s energy level doesn’t match the electron’s initial orbit, the energy released as light is less than the absorbed energy creating the first void. Thus, the wavelength of the emitted light is longer than the wavelength of the absorbed light, rendering visible light if the energy difference is in the visible range.
The process isn’t complete, because the first void still isn’t filled. A cascade of electrons fills the void and dissipates the residual energy. Ultimately, the crystal’s original state remains unchanged; the process can be repeated without changing the fluorescent material’s properties. Changing the absorbing atom’s surroundings changes the energy difference — and, thus, the wavelength and color — of the emitted light.
For example, consider the mineral zinc orthosilicate; if I place manganese and zinc atoms together, I will obtain a green light (standard-sign green). But, when I use silver instead of manganese, the reaction emits blue light, because the electrons within silver atoms exhibit different energy levels in their orbits than manganese electrons.
Conversion efficiency
The conversion efficiency and, thus, a neon tube’s total light output at a given electrical input, is based upon the probability that every UV lightwave will be absorbed into the fluorescent powder and deliver the desired process. A precise energy-level ratio must exist between incidental lightwaves, and absorbing and emitting atoms.
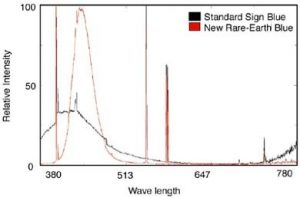
Ideally, the emitted light generates only a single wavelength, wherein all energy transforms into the single color, and provides the highest efficiency and most intense color. But, in reality, wasted energy is expended, which somewhat limits color brilliance. Fig. 3 demonstrates standard-sign blue, whose chemical name is calcium tungstate.
The benefits of fluorescence
Physicists have researched fluorescent materials, in an effort to engineer materials with defined energy levels, that confine emitted energy into a narrow band of unmistakable color (Fig. 3). This graph is normalized to the total light output; thus, you can see that the new, rare-earth blue emits in a very narrow band, with its total intensity much more concentrated. This yields a more powerful, visible impact.
Three high-intensity, rare-earth phosphors mix to yield triphosphor whites, which concentrate energy into three narrow bands of red, green and blue (similar to TV screens) to create a white wavelength. These new, fluorescent materials produce much brighter whites than traditional halophosphate whites. By contrast, unstable halophosphates distribute energy in a wide wavelength range.
But, these very intense, narrow-band emitters may create problems backlighting digital prints, or in applications that require a high, color-rendering index (see ST, August 2002, page 20). Therefore, the sign industry has developed fluorescent materials that provide very good color rendering at high intensity without reaching the extremely high lumen output of narrow-band, rare-earth, triphosphor whites.
Parting thoughts
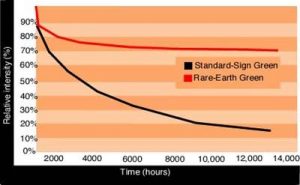
Some "old-school" fluorescent materials deteriorate quickly, whereas new materials hold up quite well inside a neon tube. Fig. 4 shows the contrasting, light-output decay of standard-sign green — the oldest material still in use — and a modern, rare-earth-green fluorescent powder.
Some say a lamp’s useful life ends when 50% of its initial intensity is lost. Thus, the old sign-green tubes must be replaced after roughly 5,000 hours (although such green signs last sometimes more than 50,000 operating hours). Rare-earth green maintains more than 75% of its initial intensity well after more than 15,000 hours. The experimental data noted here was a different estimate than many calculations conveyed in various lightsource ads.
Summarily, careful engineering has increased neon-tube fluorescent coatings’ luminous efficiency an average of 15 to 20%, and has stemmed light-output loss quite adeptly.


 Tip Sheet4 days ago
Tip Sheet4 days ago
 Business Management2 weeks ago
Business Management2 weeks ago
 Women in Signs2 weeks ago
Women in Signs2 weeks ago
 Real Deal5 days ago
Real Deal5 days ago
 Benchmarks1 day ago
Benchmarks1 day ago
 Editor's Note1 week ago
Editor's Note1 week ago
 Line Time2 weeks ago
Line Time2 weeks ago
 Product Buying + Technology1 week ago
Product Buying + Technology1 week ago